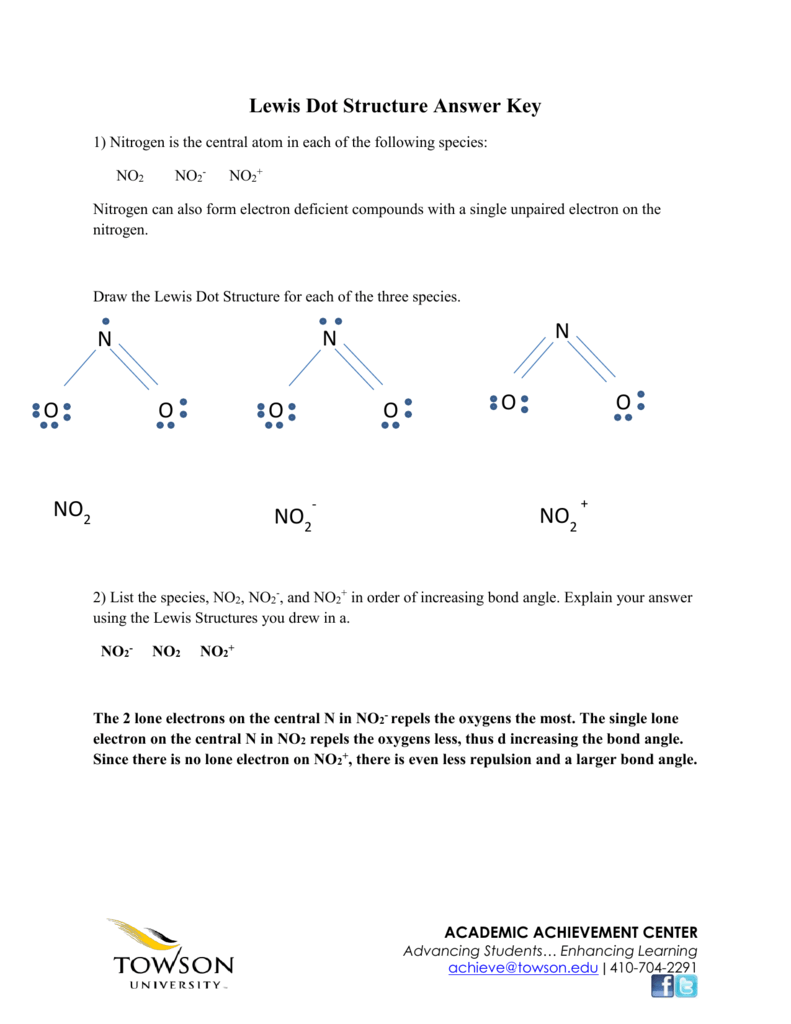
Answer Key
Unlike polar bonds, non-polar bonds share electrons equally. A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in electronegativities that is less than 0.4. An example of a non-polar bond is the bond in chlorine. Chlorine contains two chlorine atoms.

Is NO2+ (Nitronium Ion) Polar or Nonpolar? YouTube
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Is N2O Polar or Nonpolar? YouTube
Published by on NO2 polar or nonpolar - No2 is an organic chemical compound. the chemical name of no2 is nitrogen dioxide. and no2 is polar molecules. Hello, reders welcome to another fresh article, today, we will discuss about no2 polar or nonpolar and charge, molar mass, structure . We provide valuable information regarding this topic.

Draw Lewis structure for NO2^ + (nitronium ion).
On the other hand, non-polar molecules are those where atoms shared an equal number of electrons or where polarity will be canceled out in a molecule. In the NO2 molecule, the oxygen atom is more electronegative than the nitrogen atom, hence the oxygen atom pulls electrons towards itself. Therefore, the N-O bond is polar in nature.

Is the Nitronium Ion (NO2+) Polar or Non Polar? Lewis Structure YouTube
Learn to determine if NO2 + is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like NO2+ are sometimes confusi.

Poláris és nem poláris molekulák Free Press
Page Contents show What makes a molecule polar or non-polar? A molecule is polar if there is a non-uniform charge distribution present in it. If the charge distribution gets equally balanced in different parts, then that molecule or molecular ion is considered non-polar.

Science Coverage Is NO2+ Polar or Nonpolar? Electron affinity
Home Chemistry Is NO2+ Polar or Nonpolar? by Richard - October 07, 2020 0 NO2+ is a nonpolar molecule despite two N-O bonds are polar. NO2+ has a linear geometry due to no lone pair of electrons on central N atom which causes cancellation of both positive and negative charges produced on the molecule, as a result, the net dipole becomes zero.

Is NO2 Polar or Nonpolar? (Nitrogen dioxide) YouTube
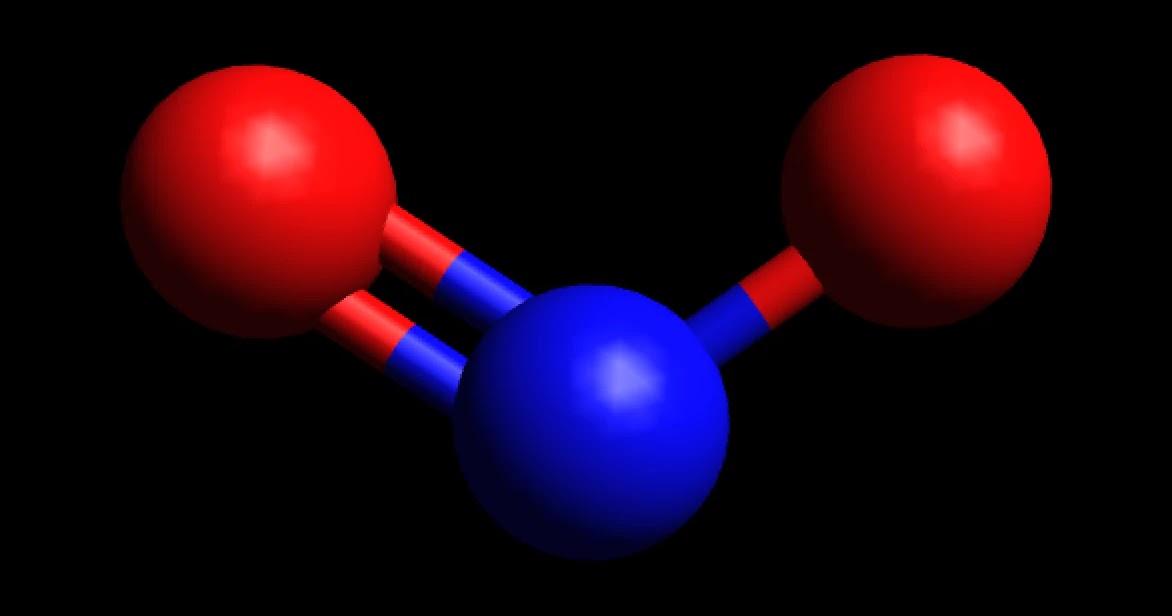
Want to know the reason? Let's dive into it! NO2 is a POLAR molecule because it has one unpaired electron on the Nitrogen atom (N) which causes the entire molecule to bend. This bending of NO2 molecule results in asymmetric geometry, which makes the molecule polar.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. As a result, both atoms have equal electronegativity and share an equal proportion of charge and the overall molecule result in a net-zero dipole moment making it a nonpolar molecule. Nitrogen, or N2, is a very abundant and necessary chemical.

ハンドメイ No.2の通販 by DREAM STAR's shop|ラクマ カテゴリ
Because the two C=O C = O bonds in COX2 C O X 2 are polarized (whereas in OX2 O X 2 the bond is not polarized) it makes it easier for the polar water molecule to solvate it and to form hydrogen bonds. Both of these factors will stabilize a COX2 C O X 2 molecule more than an OX2 O X 2 molecule in water; stabilization translates into greater.
MakeTheBrainHappy Is NO2 Polar or Nonpolar?
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Is nitronium NO2+ ion polar or nonpolar? YouTube
Therefore, a N-O bond is polar with the oxygen negative and the nitrogen positive. If the NO2 molecule were linear, the polar bonds would oppose each other and the molecule would be non-polar. Instead, a non-bonding pair of electrons are on nitrogen. The pair of electrons repel the bonds and distort the molecule into a bent geometry.

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
NO2 is a polar molecule and the Oxygen atom closest to negative side as the electronegativity of Oxygen (3.44) is comparatively greater than Nitrogen (3.04) so that Nitrogen has a partial positive charge and Oxygen has a partial negative charge established within the molecule.

Is NO2 Polar Or NonPolar? NO2 Charge, NO2 Structure, NO2 Molar Mass
Is NO2 Polar or Nonpolar? (Nitrogen dioxide) Wayne Breslyn 724K subscribers Join Subscribe Subscribed 98 16K views 2 years ago Learn to determine if NO2 (Nitrogen dioxide) is polar or.

Best overview Is NO2+ Polar or Nonpolar 1
If you look at the Lewis structure for N2O it appears to be a symmetrical molecule. However, to determine if N2O is polar we consider the molecular geometry.

Difference between polar and nonpolar examples
Geometry NO2 Polar or Nonpolar To determine if NO 2 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Nitrogen is the central atom, so we can draw the skeletal structure: